AsahiKASEI Glycated Albumin reagent
Overview
Lucica® GA-L is supplied as a ready-to-use, liquid reagent kit, for the measurement of glycated albumin, which contains high-specificity protease and enzymes, and can be utilized with general biochemical automated analyzers, and delivers same-visit results prior to physician consultation, and employs a novel BCP method that is highly specific to albumin.
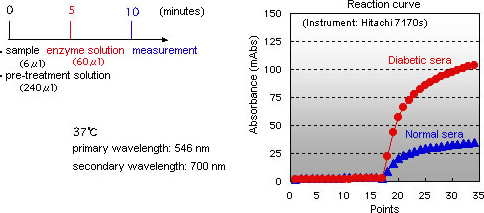
Assay Principle
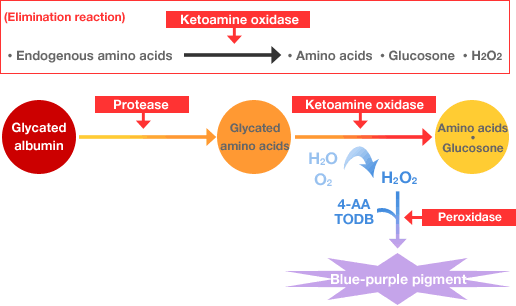
Glycated albumin: Influence by endogenous glycated amino acids is avoided through the use of an elimination reaction.
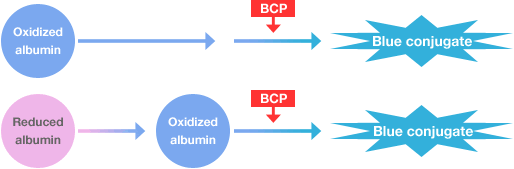
Albumin: A novel BCP method permits more specific measurement of albumin.
Calculation of glycated albumin (GA) value

Kouzuma T, et al. Clinical Chimica Acta 346:135-143 (2004)
Assay Precision
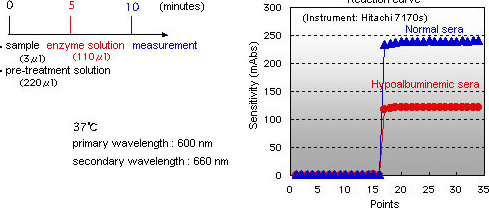
Intra-day reproducibility(n=20)
| Control | Normal sera | Diabetic sera | |
|---|---|---|---|
| Mean | 14.4% | 15.9% | 24.5% |
| SD | 0.09 | 0.13 | 0.23 |
| CV | 0.63% | 0.82% | 0.93% |
Inter-day reproducibility(n=20)
| Normal sera | Diabetic sera | |
|---|---|---|
| Mean | 16.2% | 24.8% |
| SD | 0.091 | 0.166 |
| CV | 0.56% | 0.67% |
Reduced Frequency of Calibration
| Normal sera | Diabetic sera | |
|---|---|---|
| Mean | 15.9% | 24.3% |
| SD | 0.109 | 0.182 |
| CV | 0.68% | 0.75% |
A CV of less than 1% was attained even though calibration was conducted only on the first day of a two-week period of assays.
Interfering Substances
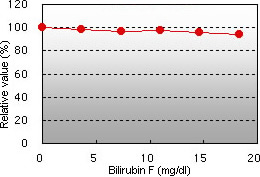
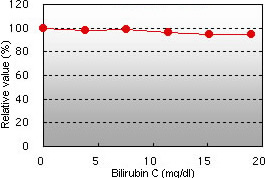
Bilirubin F interference Bilirubin C interference
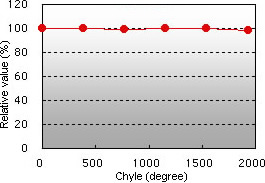
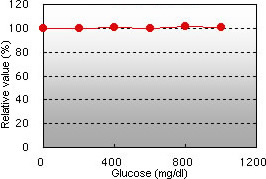
Chyle interference Glucose interference
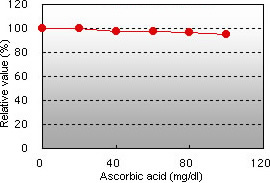
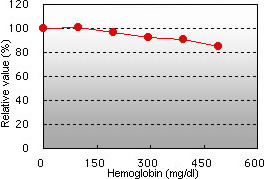
Ascorbic acid interference Hemoglobin interference
Bilirubin F and C, chyle, and glucose demonstrated almost no interference in the glycated albumin (GA) assay.
Ascorbic acid up to 100 mg/dL and hemoglobin up to 196 mg/dL demonstrated no interference in the GA assay.
Hemoglobin demonstrated slightly negative interference.
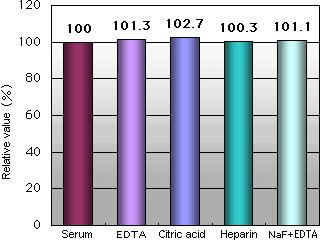
Anti-coagulant/Glycolytic Inhibitor Interference
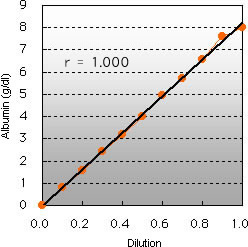
Linearity

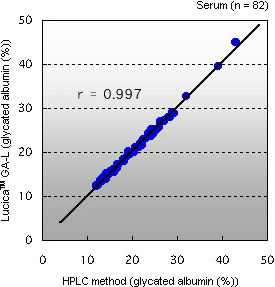
Correlation
Assay Examples
Glycated Albumin Reagents
Albumin Reagents